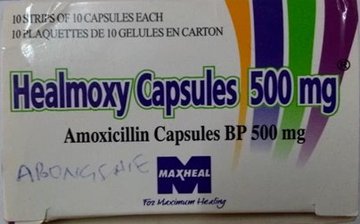
The product, Healmoxy 500mg Capsules, contains Amoxicillin Trihydrate, a broad spectrum antibiotic used to treat a wide range of bacterial infections including respiratory, urinary tract, skin, and gastrointestinal infections.
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert regarding the circulation of falsified batches of Healmoxy Capsules 500mg, which were reportedly discovered in Cameroon and the Central African Republic (CAR).
The affected product, manufactured by Maxheal Pharmaceuticals (India) and registered in Nigeria by Nkoyo Chemist, based at 23 Agbonyi Street, Aguda, Surulere, Lagos, has been flagged for lacking its active pharmaceutical ingredient (API) and carrying inconsistent manufacturing and expiry date formats, according to laboratory analysis by the World Health Organisation (WHO).
The product, Healmoxy 500mg Capsules, contains Amoxicillin Trihydrate, a broad spectrum antibiotic used to treat a wide range of bacterial infections including respiratory, urinary tract, skin, and gastrointestinal infections.
NAFDAC clarified that an investigation by Pharmaceutical Services, Lagos into the importation records of Nkoyo Chemist Ltd revealed no evidence that the affected batches were brought into Nigeria.
Nonetheless, the agency is urging importers, distributors, healthcare professionals, and the general public to remain vigilant.
All pharmaceutical products must be sourced from authorised and licensed suppliers, and their physical integrity and authenticity should be verified before use.


FOLLOW US ON:
ADVERTISE WITH US:









